The pH is a logarithmic measure of the concentration of free hydrogen
ions in a chemical or biological system. It's a very important concept
in cell biochemistry. Titratable acid, on the other hand, is a simple
measure of the (related) amount of acid 'anions' in a juice. There is
no direct relationship
between
titratable acidity and pH in apple juice, although generally the pH
goes up as
the acid goes down and vice-versa. The exact relationship differs from
sample
to sample and depends on esoteric concepts like 'buffering capacity'
which will
vary for a whole host of reasons. Nevertheless some general
empirical
relationships may be obtained, and some that I've tabulated are shown
below. It
must be stressed that they only refer to those fruits growing in those
locations at that time. They cannot be held to be exact in other
circumstances. A more detailed exploration, with additional data, is given at the end of the page.
In general, titratable acid (TA) relates pretty well to the
'acid
taste' of
a juice or cider. If the TA doubles, people will tend to
perceive
it as
twice as acidic. The pH relates more to things like microbial
stability
and susceptibility to mould and bacterial spoilage. In particular, the
antimicrobial
effectiveness of sulphur dioxide is very
pH
dependent (see the sulphite page), and this is the reason that commercial
cidermakers
measure it (see Jarvis
and Lea 2000.) TA is measured by titration, and pH by a pH
meter or
by
narrow-range pH test strips*. A pH meter is tricky to set up
and
calibrate, and only really worthwhile if it's being used daily in a
laboratory environment. The cheap pH 'dipsticks' do not have
replaceable electrodes and may only have an effective life of a year or
so. For most non-commercial cidermakers, I used to recommend pH test strips as a
better bet. But now after doing a formal comparison in 2011 I'm not so sure. Read what I found here at pH measurement comparisons. If you want to measure titratable acidity, you
can
get kits from home winemaking suppliers or from Vigo.
Or you can 'do it yourself' from my titration page here.
* [eg Merck indicator strips pH 2.5 - 4.5 (Merck product code 109451; VWR catalogue number 31501). A more readily available alternative may be the 'Vinoferm pH strips 2.8 - 4.6' available in the UK from the Home Brew Shop see http://www.the-home-brew-shop.co.uk/acatalog/Acid_Testing_Equipment.html or elsewhere in Europe from Brouwland http://www.brouwland.com as catalogue no 013.073.2. (For distributors elsewhere in the world, check out the links on the Brouwland website)]
Note that the Vinoferm strips do not read accurately in most artificial light, only by daylight. The reference dyes show a 'metachromic shift' and will not then correspond to the colour of the test portion. But if you cannot read the strips by day, I'm advised by Rose Grant that a small halogen desk lamp (though not a regular fluorescent tube) works just as well. That's a useful tip!
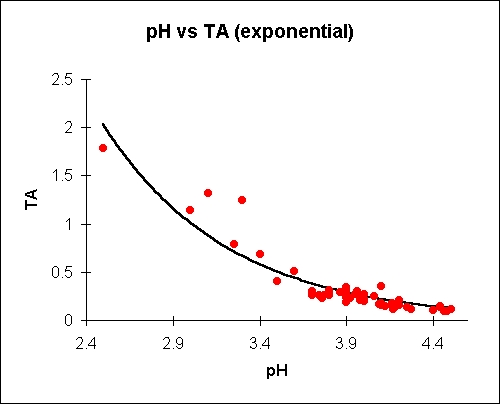
Titratable acid is given as % malic. Plots and equations
shown
are
'best-fit' to the empirical data (about 50 data points, mostly
bittersweets)
. Both binomial and exponential plots are given.

where Y is
titratable acid in %
malic and X is pH.
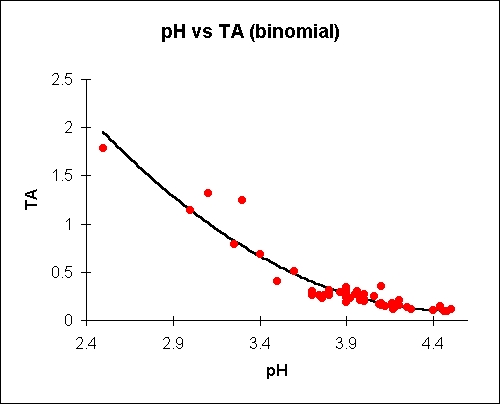
where Y is titratable acid in % malic and X is pH
In fact, because pH is a logarithmic scale, you actually get a more sensible looking 'straight-line' relationship if you plot pH vs TA on a 'semilog' plot. This is what has been done for the data below which was put together by Claude Jolicoeur in Québec from a number of sources. The straight blue line is the 'best fit' to the data, but the important thing is the two red lines which enclose the '95% confidence limits'. What this shows is that there is considerable natural variation in the relationship - for instance a juice with an acidity of 5 g/L (0.5%) could have a pH below 3.3 or above 3.9. Plainly this is not much use if you want to be within 0.1 / 0.2 pH units for SO2 dosage, for instance. Conversely, pH is little use as a measure of titratable acidity - for instance, a juice with a pH of 3.6 could have an acidity as low as 2.5 g/L or as high as 10 g/L. Hence pH and titratable acidity do not measure the same thing and you cannot get one to stand in for the other by using a fixed conversion factor.
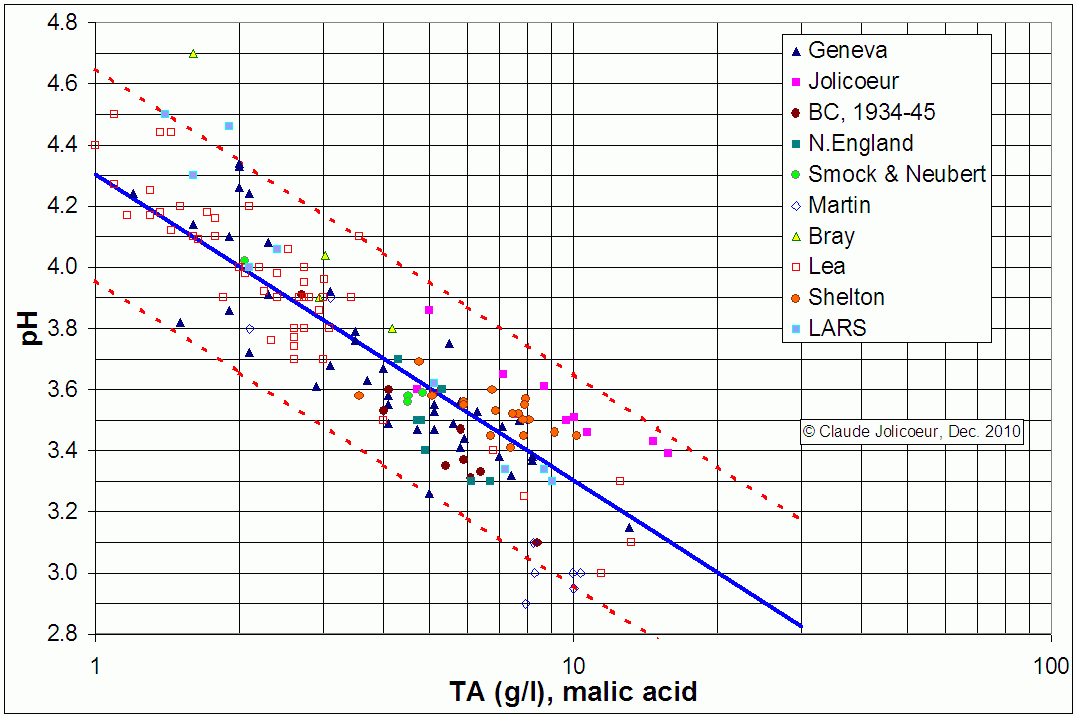
If you want to read an excellent essay on the pH / acidity relationship and for a further discussion of that data, you should download it from Claude Jolicoeur's site http://cjoliprsf.ca/Documents/Acidity-pH.pdf
Updated 31st October 2011